Abstract
Introduction:
5-azacytidine treatment prolongs survival in older patients with high-risk MDS compared to standard of care options outside allogeneic stem cell transplantation (AHSCT) wich is still the only potentially curative treatment option that is however associated with a considerable treatment-related morbidity and mortality (TRM). Reduced-intensity conditioning (RIC) prior to transplantation has broadened the application of this therapeutic approach also to elderly MDS patients.
Here we present results from a prospective multicenter trial of the German MDS and Cooperative Transplant Study Group comparing 5-azacytidine (5-Aza) treatment alone with 5-Aza induction followed by AHSCT according to donor availability in elderly patients with newly-diagnosed untreated HR MDS aged 55-70 years (EudraCT Number 2010-018467-42). Primary endpoint was overall survival (OS) at three years after 5 Aza induction in both arms; major secondary endpoints were to assess the response rate, event-free survival (EFS) at three years, toxicity, treatment-related mortality (TRM) and the impact of the comorbidity-index (HCT-CI) on outcome in both treatment arms.
Methods and Patients
Patients with proven de novo or therapy-related MDS / CMML (WBC <13 GPT/l) according to FAB and risk profile according to IPSS: intermediate II-risk or high-risk or intermediate I with high-risk cytogenetic and patients with secondary AML (according to WHO) and blasts ≤ 30 % (= RAEB-t according to FAB) and sufficient organ function, could be included.
A donor search was started at the time of inclusion and all patients were scheduled to receive 4 to 6 cycles of 5-Aza induction therapy. In case a HLA -identical sibling or a 10/10 HLA compatible unrelated donor could be identified during the first cycles of 5-Aza and patients had at least stable disease they were allocated for a busulfan-based RIC allograft. In case no suitable donor could be identified 5-Aza was continued until progression or unacceptable toxicity. This protocol-defined primary analysis uses the full 5% alpha error for the primary analysis. A final follow-up analysis will be performed once all patients have been followed-up at least for three years but no later than January 2019.
Results
Between June 2011 and November 2016 190 patients with a median age of 63 years from 14 German centers were included into the trial. Following 5-Aza induction, only 109 out of 190 patients (57%) were eligible for allocation to one of the treatment arms and proceeded to AHSCT (n=83) or continued 5-Aza therapy (n=26). Reasons for premature study termination of 81 patients (43%) within the first 5-Aza cycles included progressive disease (n=25; 31%), death (n=14; 17%), inclusion or exclusion criteria not fulfilled (n=18, 22%), withdrawal of informed consent (n=7; 9%), adverse events (n=7; 9%) or other reasons (n=10; 12%).
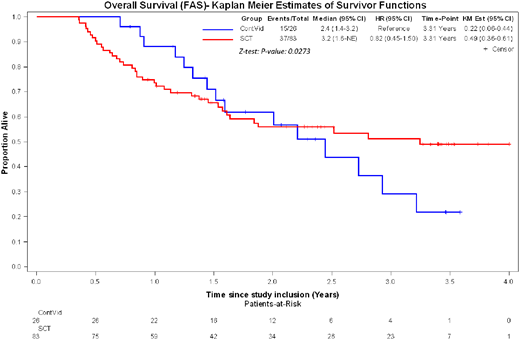
Regarding the primary study endpoint and after 5 Aza induction therapy in an intention to treat analysis the OS at 3 years was 49% (95% CI: 36-61%) after AHSCT and 22% (95% CI: 6-44%) after continuous treatment with 5-Aza (p= 0.027). The time dependent Hazard ratio for AHSCT decreased over time: while at 1 year the HR was still 1.4 due to early TRM, this HR decreased to 0.35 at 2 years and 0.09 at 3 years. EFS at 3 years was 35% (95% CI: 22-48%) after AHSCT and 0% after 5-Aza continuous treatment (p< 0.001). The TRM after AHSCT at 1 and 3 years was 17% (95% CI 10-26%) and 23% (95% CI: 14-33%), respectively.
Conclusions
This prospective phase 3 study comparing 5-Aza followed by AHSCT with continuous 5-Aza therapy in older patients (55 to 70 years) suffering from higher-risk MDS demonstrates an improved EFS and OS in favor of AHSCT. Induction therapy with 5-Aza prior to AHSCT is associated with a considerable rate of drop outs due to progression, adverse events, and mortality prior to AHSCT.
The study was registered under ClinicalTrial.gov: NCT01404741.
The study was supported by a research grant from Celgene, Germany.
Kroeger:Celgene: Honoraria, Research Funding; Neovii: Honoraria, Research Funding; JAZZ: Honoraria; Riemser: Honoraria, Research Funding; Sanofi: Honoraria; Novartis: Honoraria, Research Funding. Bethge:Miltenyi Biotec GmbH: Consultancy, Honoraria, Research Funding; Neovii GmbH: Honoraria, Research Funding. Schlenk:Pfizer: Research Funding, Speakers Bureau. Kobbe:Amgen: Honoraria, Research Funding; Celgene: Honoraria, Other: Travel Support, Research Funding; Roche: Honoraria, Research Funding. Bug:Novartis Pharma: Honoraria, Research Funding; Jazz Pharmaceuticals: Other: Travel Grant; Celgene: Honoraria; Astellas Pharma: Other: Travel Grant; Janssen: Other: Travel Grant; Amgen: Honoraria; Neovii: Other: Travel Grant. Scheid:Celgene: Honoraria; Janssen: Honoraria; Novartis: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; BMS: Honoraria; Amgen: Honoraria. Krönke:Celgene: Honoraria. Stelljes:Novartis: Honoraria; Amgen: Honoraria; JAZZ: Honoraria; MSD: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding. Beelen:Medac: Consultancy, Other: Travel Support. Platzbecker:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal